Does Green Light Make Water Evaporate Faster?
A recently
published paper in the Proceedings of
the National Academy of Sciences shows that water can evaporate faster when
exposed to green light. We decided to repeat that experiment with a green laser
and a .1 mg balance. Our preliminary data seems to show green laser light can
affect the evaporation rate of water, but not in the way you might expect.

A drop of de-ionized water was placed on a .1 mg scale.
Readings were taken every minute for six minutes under normal conditions at
room temperature and with no additional light. A green laser shown on the right
was then turned on and aimed at the drop of water, and more readings were taken
for six minutes. The laser was then turned off and another six minutes of
readings were taken. The temperature data is shown plotted on the following
graph.
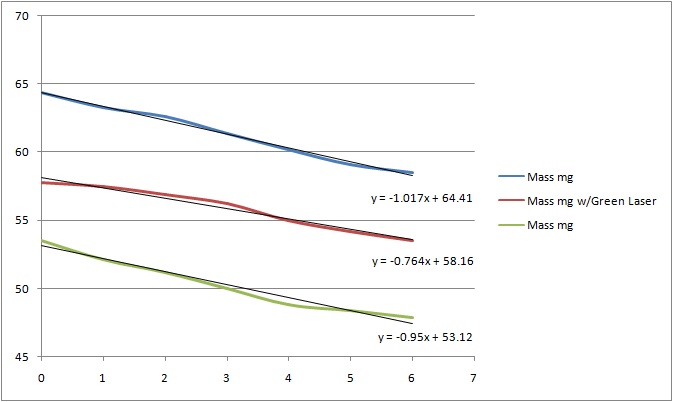
The blue line shows the mass of the drop of water decreasing
as it evaporates. We might expect the evaporation rate, the slope, to change as
the water drop size and its surface to mass ratio changes. Exposing the drop to laser light as shown
with the red line should not decrease the evaporation rate of the water drop.
Surprisingly this preliminary data shows the evaporation rate of the water
drop, the slope of the red line, decreases when the laser light is on. As a control, the laser is then turned off and
the green line shows the resulting evaporation rate.
The .39 mW laser does not have enough power to significantly
affect the evaporation rate by thermally heating the drop. Furthermore heating
the drop should increase the evaporation rate, not decrease it.
Clearly more data is needed here. The experiment should be
repeated. The angle of the laser and the focus spot on the drop should be
varied. The temperature of the water drop should be monitored. Is this a
quantum effect that structures the water molecules on the surface so that they
are less likely to evaporate? Or could it be that the surface water does
evaporate faster when exposed to green laser light, which cools the surface and
ultimately decreases the overall evaporation rate?
What do you think? Stay tuned to this website. We’ll be posting
more data and hopefully a sound explanation when we have one.
Let us know if you decide to do your own experiments and/or
have an explanation for this effect by sending us an email at
Dave@PhysicsGuy.org.

